INDICAID COVID-19 Rapid Antigen Test – The Most Economical And Effective Solution
The INDICAID COVID-19 Rapid Antigen Test (CE-IVD) is a lateral flow immunoassay designed for the qualitative detection of SARS-CoV-2 antigens in direct nasal swab samples. Antigens are present in the SARS-CoV-2 virus, and can be bound with specific antibodies.
When a virus enters a human body and begins to multiply, the body begins to react to the viral antigens, possibly resulting in symptoms.
The INDICAID COVID-19 Rapid Antigen Test Rapid detects antigens from SARS-CoV-2 virus and can be used for COVID-19 screening during active infection
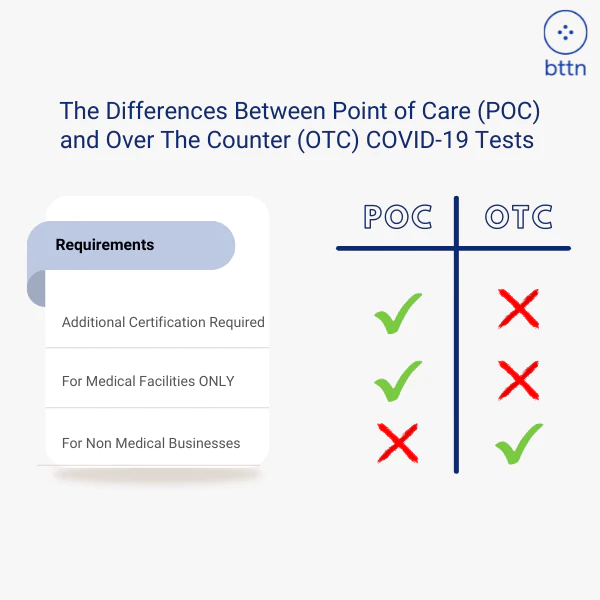
A Rapid Point Of Care COVID-19 Test
- Sensitivity and Specificity
− COVID-19 – Sensitivity 100%, Specificity 98% - FDA Emergency Use Authorization (EUA)
- Visually read in 10 minutes
For use in CLIA waived Point of Care facilities operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation

COVID-19 Test Validation
The clinical performance of the INDICAID COVID-19 Rapid Antigen Test was evaluated by testing 50 positive and 50 negative SARS-CoV-2 retrospective clinical specimens from unique donors that were previously confirmed by a molecular test.
The 100 clinical specimens were nasopharyngeal swab samples diluted in saline. Testing was performed at one investigational site by two untrained operators who were blinded to the RT-PCR results of the samples.
The samples were first randomized, then each sample eluate was inoculated onto kit-provided swabs and processed as instructed in the test procedure.
The INDICAID COVID-19 Rapid Antigen Test correctly detected 48/50 positive samples and demonstrated no false positives for the negative samples.

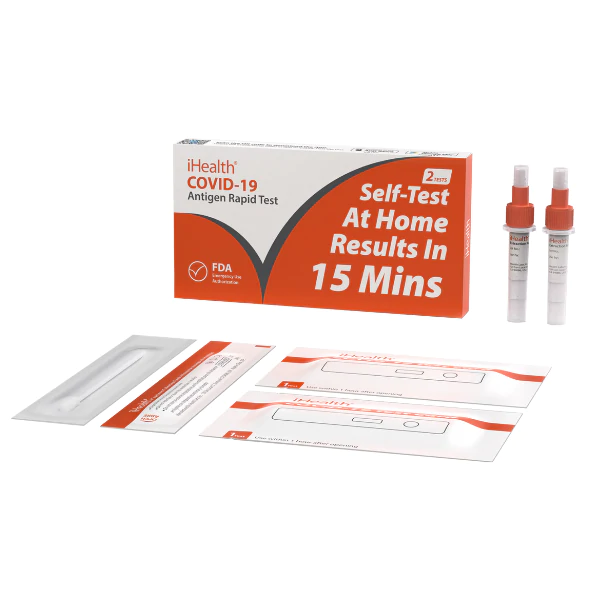
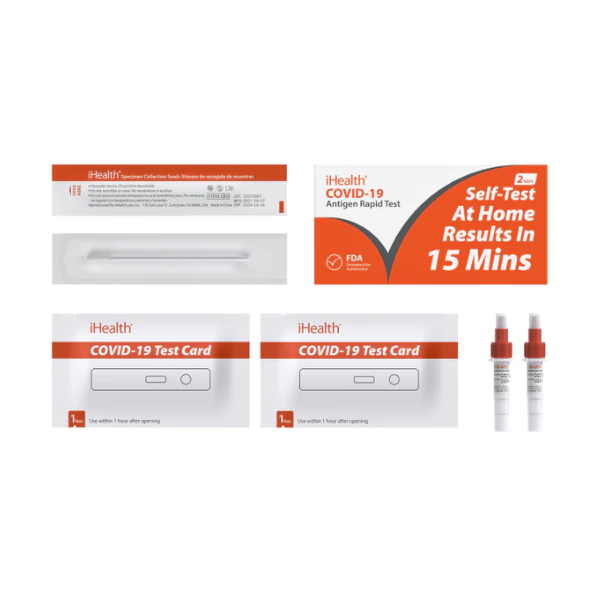
Materials Provided In Kit:
1. 25 individually wrapped Test Devices
2. 25 Buffer Solution Vials
3. 25 individually wrapped Swabs
4. 1 IFU and Quick Reference Guide
















Reviews
There are no reviews yet.